
Forest genetic resources in Nordic oak forest – current possibilities and future prospects
- Home
- Our work
- Forest
- Statistics reports and other articles
- Forest genetic resources in Nordic oak forest – current possibilities and future prospects
Oaks, renowned for their ecological importance, cultural significance, and economic value, have long been integral to the temperate forests of the Nordic countries, albeit underutilized. With the escalating challenges of climate change and its growing impacts on forest ecosystems, there is a pressing need to diversify forestry practices by incorporating a more diverse array of species, including oaks. This article aims to explore the current knowledge we have of the forest genetic resources of oak, how these resources are being used and the potential for future use across the Nordic countries.
Text by: Mari Mette Tollefsrud (NIBIO), Johan Kroon (Skogforsk), Gunnar Friis Proschowsky (Danish Nature Agency), Mari Rusanen (Luke), Oda Otilie Holltrø Spongsveen (NordGen).
The climatic conditions in the Nordic countries only provide conditions for a small number of naturally occurring forest tree species. In Finland, Sweden and Norway, forestry practices have traditionally focused on boreal conifers, such as spruce and pine, due to their adaptability and economic significance. In the Danish forestry, as well as in Islandic afforestation, a larger diversity of species has been used, however mostly in small-scale forestry. Climate change continues to alter environmental conditions, and there is a growing awareness of the need to diversify forest ecosystems across Europe (Gömöry et al., 2021). Deciduous tree species, including oaks, are gaining attention for their potential to enhance biodiversity, resilience, and sustainability in Nordic forests (e.g. Löf et al., 2012; Olofsson and Jakobsson, 2023). Oak is also especially suitable for wood production. Due to its technical qualities, oak has many valuable and refined uses, e.g. furniture production.
With climate warming, the northern distribution limits of deciduous tree species, including oak, are expected to shift northward (Mauri et al., 2022). This presents both challenges and opportunities for forestry in the Nordic region. Active forest management practices, including planting and promoting the growth of deciduous tree species, are essential to facilitate their expansion into new suitable habitats and mitigate the adverse effects of climate change on forest ecosystems. Changes in forest management are underway, with focus on biodiversity conservation, production potential, and biorefinery production. This shift reflects a growing recognition of the importance of maintaining ecosystem health while maximizing economic benefits. As part of this transition, the exploration of forest genetic resources (FGR) of oak and other noble hardwood species is crucial. Understanding the genetic diversity within these species is essential for developing informed strategies for sustainable forest management and adaptation to changing environmental conditions. This article aims to provide knowledge on forest genetic resources of oak in the Nordic region, including the potential for exchanging FRM across the region.
1. Distribution, genetic variation, and hybridization in the Nordic oak populations
Distribution and ecology
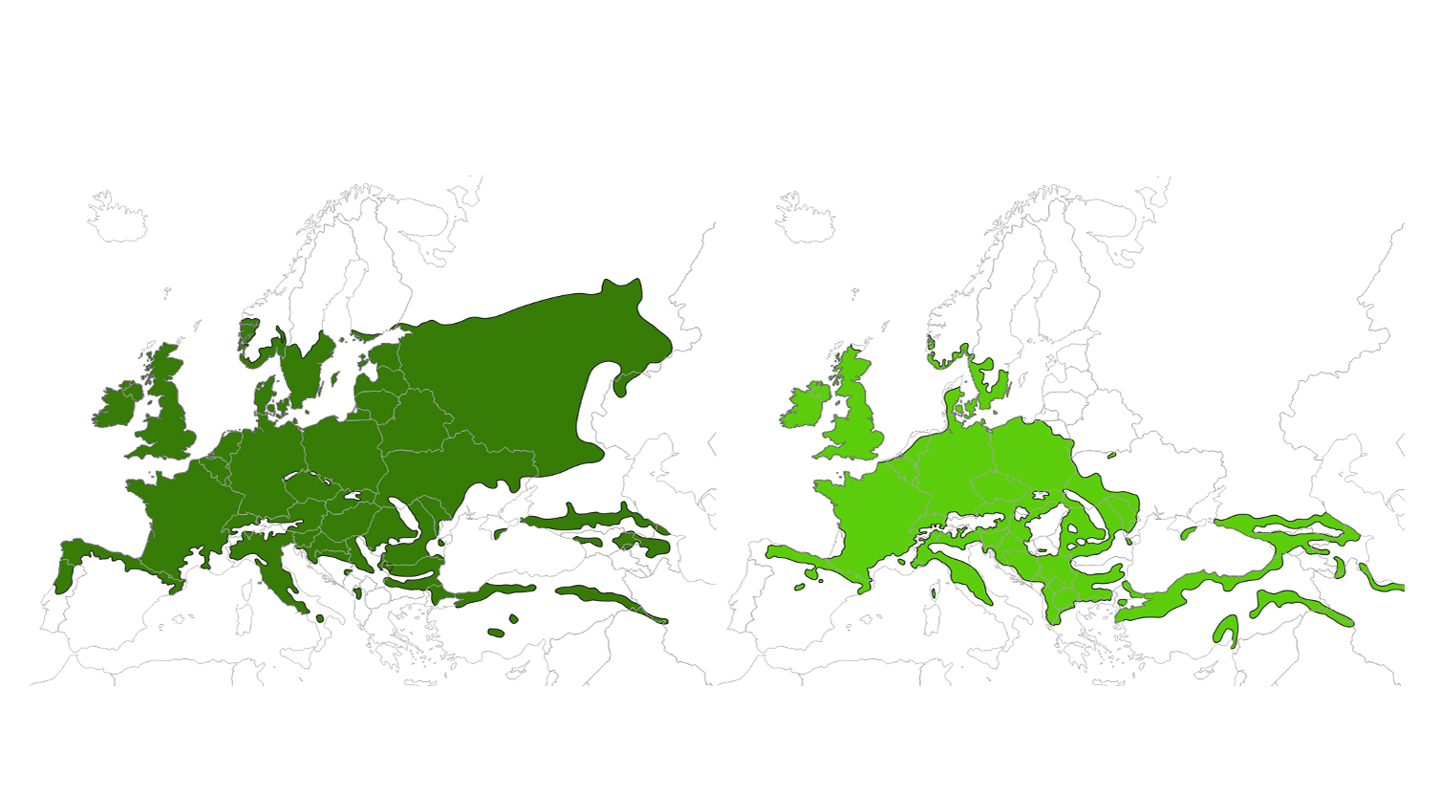
The genus Quercus comprise approximately 435 species worldwide representing a significant tree species diversity across the Northern Hemisphere (Denk et al., 2017) of which 30 occur in Europe (Kremer and Hipp, 2020). The two oak species which are relevant from a Nordic perspective are Quercus robur (pedunculate oak) and Q. petraea (sessile oak). These are temperate white oak species with a largely sympatric distribution range across Europe.
In the Nordic countries, Q. robur extends further north and to the inland compared to Q. petraea. In Sweden, Q. robur is the dominant oak species and occurs up to the natural northern limit of its range, while the Q. petraea is only present in the southernmost parts of Sweden. Denmark is within the natural range of both species where Q. robur is widely distributed and the most common. Q. petraea is not as common as Q. robur but is especially common in central Jutland. In Norway, the distribution of oak is largely restricted to the coastal areas with the core area of Q. petraea restricted to the southernmost part of Norway, Agder. In addition, scattered occurrences of Q. robur are found in the inland. In Finland, Q. robur is found in the south, whereas Q. petraea has no natural occurrence. Both species are absent from Island (Fig 1).
Within their primary distribution range, Q. petraea and Q. robur demonstrate varying ecological characteristics, showing diverse ecological affinities and functional roles. However, our understanding of their behavior in the Nordic countries is limited. Research from Europe has shown that both species have different ecological preferences and roles within ecosystems. While Q. petraea is adapted to drier conditions, tends to establish itself in later stages of succession, and forms part of mature woodland communities, Q. robur is more adaptable to various soil conditions, prefers fertile and humid soils, tolerates wetter sites, and often serves as a pioneer species in initiating ecological succession (Ducousso and Bordacs, 2004). Overall, while the ecological preferences and roles of Q. petraea and Q. robur are well-documented in the main distribution area, further research is needed to understand their behavior and contributions within Nordic ecosystems.
The impact of historical events on oaks genetic structure
During the last ice age, the oak species were largely restricted to the Iberian, Italian and eastern Balkan peninsulas, mainly in mountainous regions where precipitation was more frequent (Brewer et al., 2002). At the onset of climate warming, colonization out of the southern peninsulas was first slow in the southern ranges, then very fast across the European plains to Scandinavia and Finland. Already at 6000 before present, oaks reached their northern ranges with migration rates as high as 1000 m per year (Kremer and Hipp, 2020).
Extensive screening of populations by means of chloroplast DNA markers, which are maternally inherited and thus used to trace seed flow, has revealed the post glacial expansion routes of oaks from glacial refugia (Petit, Brewer et al., 2002; Petit, Csaikl et al., 2002). Oaks from the Italian refugia were probably the first to reach Scandinavia, the Italian oaks then migrated into Finland from Sweden across the Baltic Sea. Oaks from the Balkan refugia also reached Finland, probably via an eastern migration route. Oaks from the Iberian refugia also reached Scandinavia. In Denmark and Norway there are several oak populations that are carrying the Iberian chlorotype, in Sweden however, the Iberian chlorotype is rarer. Denmark seems to constitute a meeting zone between the different refugia (Jensen et al., 2002).
Migration during the last part of the Holocene in the northern distribution range of oaks was rapid, characterized by long-distance dispersal events giving rise to small populations characterized by single chlorotypes. Despite such repeated founder events, these long-distance dispersal events have contributed to the maintenance of genetic diversity in oak since these events have supplemented the moving colonizing front with new genetic material. Oak's longevity, their long juvenile period which allow migrants to establish, along with outcrossing, are life history traits that are important in maintaining their high level of genetic diversity.
Numerous studies have showed that oaks harbor very high levels of genetic diversity (e.g. studies based on microsatellites show very high levels of heterozygosity, studies based on single nucleotide polymorphisms (SNPS) show high nucleotide diversity; reviewed in Kremer and Hipp, 2020). A study based on exon capture sequencing also shows that oaks, as well as other tree species, have maintained their genetic diversity throughout multiple glacial cycles. Population contractions caused by ice ages do not seem to have led to a reduction in effective population size (Milesi et al., 2023). Moreover, genetic diversity in Q. petraea seem to increase with latitude (Milesi et al., 2023). The genetic resources in the Nordic oak populations are in other words very diverse, and Nordic oaks have probably high evolutionary potential for future adaptation.
Hybridization has a potential role in adaptation
Hybridization and introgression in oaks are well known, still species barriers remain despite extensive interspecific gene flow. Recent studies based on genome sequencing have shown that hybridization can occur without destroying the species integrity, even if only a part of the genome is responsible for holding up the species barriers (Leroy et al., 2017; Leroy et al., 2020). Estimates of divergence time among the European oaks vary between 1 and 5 million years (Hubert et al., 2014) and massive hybridization and introgression among the oak species Q. petraea, Q. robur, Q. pubescens and Q. pyrenaica took place during the onset of the last glacial period, before recolonization started out of the refugia (Leroy et al., 2017; Leroy et al., 2020). Notably, the chlorotypes used to trace migration routes out of the refugia, do not differentiate among species, but are shared among several species including Q. petraea, Q. robur, Q. pubescens, Q. pyrenaica (Petit, Csaikl et al., 2002).
Hybridization and introgression in the Q. robur/Q petraea complex have been identified as key factors contributing to the evolutionary success of oaks, reinforcing adaptation and enhancing migration (Kremer and Hipp, 2020). The hybridization/migration hypothesis in the Q. robur/Q petraea complex suggests the following model: First, long-distance dispersal of Q. robur nuts by jays establishes a pioneer population. Second, long-distance Q. petraea pollen flow invades the Q. robur population through hybridization. Third, backcrosses of these hybrids with Q. petraea, coupled with selection in favor of Q. petraea, allow the emergence of Q. petraea in a few generations. This process continues with long-distance seed dispersal of Q. robur followed by interspecific pollen flow into the newly formed population (Petit et al., 2004). Recent genome sequencing has corroborated this model, revealing introgression of temperature tolerance-related genes from Q. robur to Q. petraea. This mechanism likely facilitates adaptation in Q. petraea at higher latitudes and elevations (Leroy et al., 2020).
The model highlights the benefits of having both Q. Petraea and Q. robur species in terms of enhancing evolutionary potential and adaptability during expansion. In Scandinavia, both species are commonly found in the same forests, where they frequently hybridize. In Denmark, hybridization has been observed in a mixed oak stand in Velling Skov, Jutland, where a paternity analysis revealed that, on average, 85% of pollination came from fathers within the stand, with variable pollen flow directions from year to year. Inter-specific hybridization rates were high, ranging from 15-17% for Q. petraea mothers and 48-55% for Q. robur mothers (Jensen et al., 2009). Data from the EU project GenTree also indicate variable levels of hybridization in southern populations of Q. petraea in Sweden and Norway (personal communication, Pascal Milesi). However, it is noteworthy that these hybrids were not included in diversity index calculations for populations in the study by Milesi et al. (2023), referred to earlier.
2. Oak forest reproductive material (FRM) in the Nordic countries
Sweden
In Sweden, Q. robur has been the primary focus for oak silviculture. This emphasis is evident in the abundance of officially registered and approved basic material of FRM in the National List of Basic Material at the Swedish Forest Agency. For Q. robur there are about 100 seed stands and two seed orchards, whereas the number of basic materials for Q. petraea is less than 10 seed stands and no seed orchard). Most of the seed stands were originally selected in the late 19th century to the mid-20th century. By the mid-1900s, plus trees were also grafted in the plant nursery at Ekebo (now Skogforsk) and these grafts were used to establish two seed orchards with Q. robur in Skåne. One is still active and harvested during abundant acorn years.
Unfortunately, the originally approved seed stands have not been managed for seed production. They are rarely used for commercial seed harvesting in Sweden due to inadequate and unpredictable seed production. Therefore, the registered and approved FRM seed source areas for oak that have effectively become the unit for the material traded and sold in Sweden is a wider geographic area in the southern region of Sweden (Götaland).
Over the years, there has been a shortage of domestic oak reproductive material in Sweden, leading to reliance on imports, particularly from countries in central Europe. This applies, for example, to Dutch origin, which has had a good reputation and has often been used in the southernmost part of Sweden for oak silviculture. However, the transfer of material from the south can be risky. Timing of bud burst, and bud set is critical due to frost damages in spring and autumn, especially for provenances from southern Europe, which are particularly vulnerable.
Denmark
In Denmark, the stability in older oak stands is considered to be good compared to conifers and other deciduous trees. The cultivation of oak is thus also used with a purpose of capital savings because there is greater freedom to choose when a stand is felled. In Denmark, oak is also used in shelter plantations on farmlands and in forest edges. Oak from the Netherlands have been widely used due to excellent stem form and good growth. Dutch oaks are however not suitable for exposed locations in western Denmark. As for Sweden, the timing of bud burst, and bud set for these southern European provenances is critical in adaptation.
Currently 76 approved seed sources of pedunculate oak and 21 of sessile oak are present in Denmark. However, seed setting is irregular and does not allow for an even supply and therefore substantial contributions to the supply come from a smaller number of the seed sources. From 2010-2020 the Danish production was small and irregular and import of seeds and seedlings from the Netherlands and Norway was very important. For pedunculate oak 10 seed sources are approved as tested, 4 as qualified, and 62 as selected. For sessile oak 6 seed sources are approved as tested, 2 as qualified, and 13 as selected.
Norway
There is currently little oak forestry in Norway, regeneration of oak forests is mostly natural, but there is recently an increased interest in oak as a forestry species, especially Q. petraea on the south coast. The core area of Q. petraea is restricted to the southernmost part of Norway, Agder. In this area, nuts from Q. petraea seed source are collected and certified either as source identified or selected FRM. Almost all the harvested nuts are exported to Denmark. The harvested amount varies a lot from year to year. For instance, in 2020 as much as 19 495 kilos were collected and exported to Denmark. In 2021 there were no collection of nuts, but 8220 kilos were collected in 2022 and more than 99.5% were exported to Denmark. In 2023 only 5131 kg were collected of which more than 98% were exported to Denmark (Statistics from the Norwegian Forest Seed Center/Skogfrøverket).
Finland
In Finland, the Forest Act (1093/1996) defines which species can be used for establishing a forest stand, under a condition that the provenance is suitable for the site. Q. robur is listed among these species. There are six seed stands and two seed orchards officially registered as basic material. One of the seed orchards has been established with selected material for forestry purposes whereas the other is established for genetic conservation. In the later the material comes from known natural stands but is not selected for any specific traits. Although oak is suitable to be grown on the best soils in South-Finland, the use of oak in the Finnish forestry is currently insignificant. The average yearly production of plants (2006-2020) has been around 5000 plants (statistics by Ruokavirasto). The highest number of nuts collected in one year (2018) during the past 15 years has been 510 kg, 70% of which came from a seed orchard (Ruotsalainen et al., 2022).
NordGen seeds and plants report
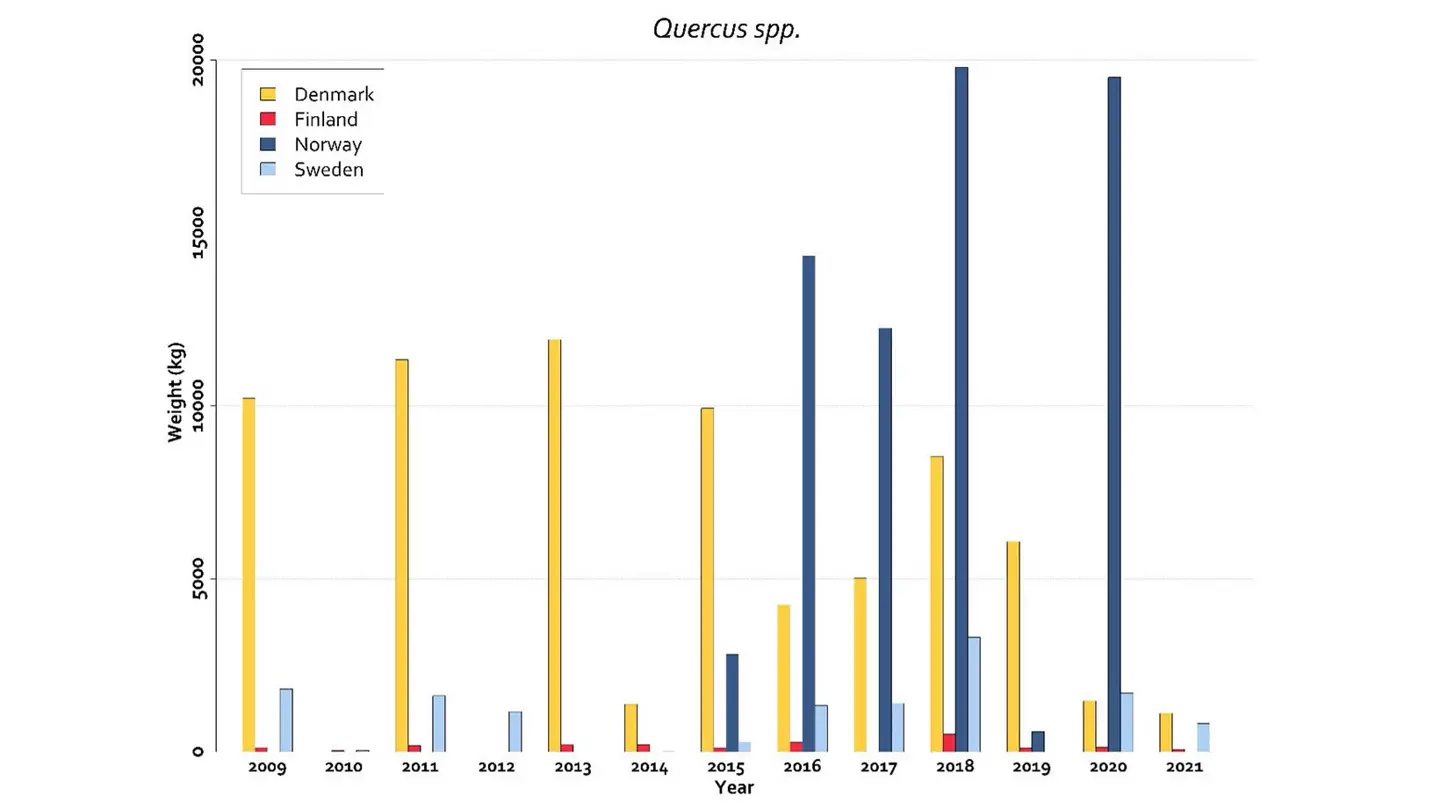
The data on Quercus spp. seed production in Nordic countries is available in the NordGen seeds and plant report (Solvin et al., 2023). The collection of oak nuts is very variable among years and countries. Prior to 2016 the largest harvests were in Denmark (Fig. 2). After several years with only minor seed years, a harvest record was in 2023, around 75 tons (data not yet available). In the period 2016 to 2020 there was on the other hand large harvests of oak nuts in Norway (Fig. 2), of which almost everything was exported to Denmark (Statistics from the Norwegian Forest Seed Center/Skogfrøverket).
3. Genetic trials of oak in the Nordic countries
The number of genetic trials and breeding activities on oak in the Nordic countries is modest, still some information is available from the different countries. Below is an overview of the main findings.
Sweden
In Sweden, forest tree breeding commenced in the mid-1930s, with a primary focus on deciduous species such as birch, aspen, and oak. Notably, two seed orchards dedicated to Q. robur were established in southern Sweden as early as the 1950s, utilizing plus trees selected in southern Sweden. However, as interest in deciduous trees waned, attention shifted towards native conifer species.
The issue of provenance has not been thoroughly investigated in trials conducted in Sweden. However, the Forestry Research Institute of Sweden (Skogforsk) has recently assessed data from Swedish gene banks containing families from Swedish oak provenances, potentially shedding light on this matter. In 1995, six gene bank sites were established in Götaland. These sites were primarily designated for the conservation of native oak in ex situ archives. Nonetheless, they also serve as valuable resources for provenance and other oak genetic research. The material housed within these sites comprises approximately 20,000 offspring sourced from over 500 high-quality oak trees with diverse local origins. Presently, four such sites remain operational in Skåne, Jönköping, Västergötland, and Södermanland. Notably, there is no other trial series in Sweden featuring oak material of comparable breadth sourced from various provenances.
It wasn't until the late 1990s that the first progeny trials utilizing clones from the initial Q. robur seed orchards were initiated. Subsequent funding injections aimed at enhancing oak breeding material between 1995 and 2001 facilitated the selection of additional elite clones for a new, smaller seed orchard located in Skåne (Trolleholm). The clones gathered from this effort were then to be tested in offspring trials conducted around the turn of the century. Notably, this expansion of the breeding population was carried out in collaboration with Denmark.
Presently, Sweden is actively engaged in establishing new oak progeny trials with both Q. robur and Q.petraea, with the most recent being launched in 2020. These endeavors signify ongoing efforts to improve and refine oak breeding programs in Sweden, ensuring the continued advancement of forestry practices and the sustainability of forest ecosystems.
Denmark
A large number of provenance trials form the background for the use of oak in Denmark. The earliest trials are from around 1900. Back then the statistical tools were not very well developed, nevertheless they have contributed to enhanced knowledge about the suitability of provenances from several countries. Almost 100 Danish provenances are included as well as provenances from the Netherlands (11), Norway (7), Germany (5) Sweden (3) and several other countries. (Larsen et al., 1997). From these provenance trials, differences in performance of provenances are documented. Focus has been on stem straightness, volume production, epicormic shoots, lammas shoots and good resistance to frost damages, pests, and fungi.
In the eastern parts of the country where wind damage and frost are less pronounced, the purpose of oak planting is forest production. Here, pedunculate oak provenances from the Netherlands are very productive and characterized with excellent stem form. In the western parts of Denmark, characterized by greater exposure to wind and frost the purpose of oak planting is often protective forests. Danish provenances of both species alongside with Norwegian sessile oak provenances from the Agder coast are recommended. At Videntjenesten you can read more about the recommendations of different oak provenances for Denmark. In the Danish Plantevalg.dk you will find an overview of Danish seed sources of oaks, as well as other tree species.
In Denmark, the oak breeding program is from the 1990s and is based on material originating from the Netherlands and Denmark. Plus-trees have been selected and offspring have been planted in several seedling seed orchards. Moreover, heritability and breeding values have been calculated for relevant properties, most importantly for growth and health related traits.

Norway
In Norway, there is one provenance trial of oak (Skrøppa et al., 2017). This trial was established in 2001 at Kaldvell, Agder. The trial has progenies from 14 Norwegian populations and three Danish populations of which one of the Danish populations had its origin in Arendal, Norway. The same material is also planted in Denmark. The trials show that there are considerable genetic differences in several traits among the different populations highlighting the importance of choosing FRM material carefully for establishing plantations with oak. A considerable amount of variation in height growth was evident among the populations. In the Norwegian trial the best performing populations were the ones from southwestern Norway (Mandal and Birkeland) together with the population from Denmark originating from Arendal, Norway. In the Norwegian trial, trees from populations with a larger percentage of Q. robur have later bud flushing, poorer height growth and poorer stem straightness (Skrøppa et al., 2017).
Norway does not have a breeding program on oak, neither plans for establishing one. However, the Norwegian Forest Seed Center has just released a revision of the national breeding strategy, where a need for developing reproductive material of oaks is identified.
Finland
There has been very little research on oak provenances in Finland. Hautsalo et al. (2015) analyzed growth and survival of five Finnish origins of Q. robur, in six field trials located in southern and central Finland, at the age of 13 years. This study revealed a significant effect of origin, survival differing more than height among the origins. They also observed a significant family(origin)*trial interaction for survival but limited local adaptation for growth.
Finland has not identified any need for a breeding program on oak. However, in the national program for establishing seed orchards a potential need to increase the seed production capacity for oak has been identified, based on a questionnaire for a specific group of stakeholders, on the priority of different species in the future Finnish forestry (Antola et al., 2023).
Nordic oak FRM, we still know little about its transferability across different climates
Genetic trials conducted in the Nordic countries, along with studies involving 33 provenances in Danish field trials (Jensen, 2000) and a European investigation of 116 populations in 23 trials (Sáenz-Romero et al., 2017), reveal substantial genetic variation in growth-related traits among Nordic oaks, indicating their adaptive potential. Eriksson (2015) consolidates this research, offering recent genetic insights into Q. petraea and Q. robur, elucidating their genetic composition and its implications for their biology and ecology in Nordic regions. However, it's worth noting that genetic research on oaks within the Nordic countries is relatively limited compared to continental Europe.
The timing of bud flush in spring and bud set in autumn are two heritable traits linked to adaptation (Eriksson, 2015). In southern and central Europe, populations from lower latitudes or elevations tend to flush earlier than those from higher latitudes and higher elevation (Ducousso et al., 1996; Firmat et al., 2017). Conversely, in northern Europe, the relationship between bud flush and latitude is more nuanced, with northern populations exhibiting earlier bud flush compared to southern populations (Jensen, 2000; Skrøppa et al., 2017), making them more susceptible to early spring frosts. Eriksson (2015) emphasizes the strong genetic control over flushing and consistent timing among populations despite significant yearly variation. This observation aligns with many studies on northern European tree species, indicating earlier flushing in northern populations compared to southern ones. An intriguing finding is the distinction between continental and coastal populations, with continental populations flushing earlier than coastal ones (Ducousso et al., 1996; Jensen, 2000). The results suggest significant interaction between different oak species and the environment. Moreover, while Q. robur demonstrates adaptability to various soil conditions and moisture levels, Q. petraea is better suited to drier sites.
In Denmark, there are trials containing the widest range of provenances, making it the most comprehensive collection of material. However, extrapolating their performance to the neighboring Nordic countries is challenging due to the lack of identical material in those regions. However, Dutch oak in southern Sweden and eastern Denmark, where exposure is lower, shows promising results for forest production, indicating successful south-to-north transfer. Still, caution needs to be taken to avoid frost damages in spring and autumn with such a south-north transfer (“assisted migration”). Additionally, experience suggests the Agder provenance from Norway thrives in exposed locations in Denmark, demonstrating a successful north-to-south transfer for specific sites.
To gain a better insight into the variation in growth traits and phenology, in oaks, there are now plans for establishing new trials across Europe in the EU funded OptFORESTS project.
OptFORESTS (2022-2027) is composed of 19 European partners. This project aims to support the conservation and sustainable use of FRG through, among other, establishment of 28 next-generation common garden trials with both provenance and species mixtures. All the trials will include Q. petraea and give very valuable information about the range wide genetic variation in growth variability and adaptation. In the Nordic countries, trials including Q. petraea will be established in Denmark, Sweden and Norway. These trials will be important to lay the foundation for developing guidelines on the use and exchange of FRM across the Nordic countries. Moreover, some of the old oak trials will also be analyzed in OptFORESTS and will give valuable information applicable to the Nordic context. Other trials that should be looked into are the TREEDIVNET experiments where oaks are planted in several of the trials (Depauw et al., 2024).
4. Concluding remarks
There are significant variations in the need for FRM of oak and other noble hardwood species among different countries in the Nordic region. Factors such as ecological conditions, historical land use practices, economic priorities, as well as policy development influence the demands for FRM. Some countries may prioritize conservation efforts and promote sustainable management of native oak populations, while others may focus on incorporating oak genetic resources into afforestation and reforestation programs. Overall, the exploration of forest genetic resources, coupled with changes in forest management practices, plays a crucial role in ensuring the resilience and long-term sustainability of Nordic forests in the face of climate change and other environmental challenges.
In areas where Norway spruce is struggling due to drought, oak may become an important supplement along with other species. From the available knowledge and literature, it is very clear that there is a large amount of genetic variation in the Nordic oaks, demonstrating their adaptive potential and relevance for the future. Since adaptation and migration seem to be fueled by hybridization and the northern populations of oaks entail both Q. robur and Q. petraea along with their hybrids, these populations may be especially interesting from a gene resource perspective.
Although some insight on transferability can be deduced out of existing provenance trials, research is needed to get more knowledge about the variation in performance of both species as well as among oak provenances and how the most important adaptive and growth-related traits vary along with climate, soil, and environment, before more general recommendations on transferability across the Nordic countries can be developed. Still, we have some experience suggesting that a south north transfer (from the Netherlands to southern Sweden and Denmark) is beneficial for forest production. More research is on its way related to adaptability of oaks across Europe through the OptFORESTS project. Nordic cooperation e.g. NordGen is important for knowledge exchange across the region and to contribute to the transfer of research into practice.
NordGen activities to facilitate knowledge exchange and cooperation across the Nordic countries
In August 2023, a workshop was organized in Arendal, Agder, southern Norway, by the NordGen working group of genetic resources together with the county manager of Agder. This event brought together stakeholders and representatives from the Nordic countries, to address the nuances of oak forestry and the utilization of FRM. The discussion highlighted several key challenges and considerations for the sector:
One of the distinct aspects of oak forestry is its extended rotation period – from regeneration to harvest – which mean that the investments made by current forest owners only will be realized by future generations. Additionally, in many regions there is a notable lack of practical knowledge regarding the production of quality oak timber. Facilitating a knowledge exchange among Nordic forest owners could be beneficial in bridging this gap. With the current timber prices, the harvesting of oak forests will for many forest owners be a break-even project. Currently, oak forestry, at least in southern Norway, is more driven by passion for cultivating beautiful and ecological valuable woodlands than by profitability. There were however no doubts that oak timber of the right quality is a product at high demand.
The workshop also discussed critical factors necessary for establishing oak forests, focusing on:
- Careful selection of the genetic material/origin to ensure that the chosen FRM matches the specific climatic conditions at the planting site.
- Careful consideration of seedling quality when planting to promote robust growth and development.
- Planting of oaks only in appropriate soil to ensure optimal development.
- When natural regeneration is chosen, it is important to maintain suitable light conditions and cultivation methods, to allow for seed development and successful germination within existing stands.
By addressing these elements, the workshop contributed to knowledge exchange and communication of oak forestry and its future potential across the Nordic region.


References
Antola J, Haapanen M, Himanen K, Leinonen K, Paanukoski S, Stenvall N. Metsänjalostuksen hyödyt käytäntöön – metsäpuiden siemenviljelysten perustamisohjelma 2060. Maa- ja metsätalousministeriön julkaisuja, 2023:19 http://urn.fi/URN:ISBN:978-952-366-719-8
Brewer S, Cheddadi R, de Beaulieu JL, Reille M, Contributors D. (2002). The spread of decidious Quercus throughout Europe since the last glacial period. Forest Ecology and Management, 156, 27-48.
Caudullo G, Welk E, San-Miguel-Ayanz J. (2017). Chorological maps for the main European woody species. Data in Brief, 12, 662-666. https://doi.org/10.1016/j.dib.2017.05.007
Depauw L, De Lombaerde E, Dhiedt E, Blondeel H, Abdala-Roberts L, Auge H & Barsoum N et al. (2024). Enhancing Tree Performance Through Species Mixing: Review of a Quarter-Century of TreeDivNet Experiments Reveals Research Gaps and Practical Insights . Current Forestry Reports, 10(1),1 -20. https://doi.org/10.1007/s40725-023-00208-y https://treedivnet.ugent.be/index.html https://treedivnet.ugent.be/index.html
Ducousso A, Guyon JP, Kremer A. (1996). Latitudinal and altitudinal variation of bud burst in western populations of sessile oak (Quercus petraea (Matt) Liebl). Annales des Sciences Forestières, 53, 775–782. https://doi.org/10.1051/forest:19960253
Ducousso A, Bordacs S. (2004). EUFORGEN Technical Guidelines for genetic conservation and use for pedunculate and sessile oaks (Quercus robur and Q. petraea). International Plant Genetic Resources Institute, Rome, Italy. ISBN 92-9043-660-3
Eriksson G. (2015). Quercus petraea and Quercus robur: recent genetic research. https://doi.org/10.20315/SFS.146.
Firmat C, Delzon S, Louvet JM, Parmentier J, Kremer A. (2017). Evolutionary dynamics of the leaf phenological cycle in an oak metapopulation along an elevation gradient. Journal of Evolutionary Biology 30(12), 2116-2131. https://doi.org/10.1111/jeb.13185
Gömöry D, Himanen K, Tollefsrud MM, Uggla C, Kraigher H, Bordacs S, Alizoti P, A’Hara S, Frank A, Proschowsky GF, Frýdl J, Geburek T, Guibert M, Ivanković M, Jurše A, Kennedy S, Kowalczyk J, Liesebach H, Maaten T, Pilipović A, Proietti R, Schneck V, Servais A, Skúlason B, Sperisen C, Wolter F, Yüksel T & Bozzano M. (2021). Genetic aspects linked to production and use of forest reproductive material (FRM): Collecting scientific evidence for developing guidelines and decision support tools for effective FRM management. European Forest Institute, EUFORGEN Secretariat.
Hautsalo J, Mathieu P, Elshibili S, Vakkari P, Pulkkinen P. (2015). Variation in height and survival among northern populations of pedunculate oak (Quercus robur L.): results of a 13-year field study. Silva Fennica, 49(2), 1-12. https://doi.org/10.14214/sf.1274
Hubert F, Grimm GW, Jousselin E, Berry V, Franc A, Kremer A. (2014). Multiple nuclear genes stabilize the phylogenetic backbone of the genus Quercus. Systematics and Biodiversity, 12(4), 405-423. https://doi.org/10.1080/14772000.2014.941037
Jensen JS. (2000). Provenance variation in phenotypic traits in Quercus robur and Quercus petraea in Danish provenance trials. Scandinavian Journal of Forest Research, 15(3), 297-308. https://doi.org/10.1080/028275800447922.
Jensen JS, Gillies A, Csaikl U, Munro R, Madsen SF, Roulund H, Lowe A. (2002). Chloroplast DNA variation within the Nordic countries. Forest Ecology and Management, 156(1-3), 167-180. https://doi.org/10.1016/S0378-1127(01)00641-7.
Jensen J, Larsen A, Nielsen LR, Cottrell J. (2009). Hybridization between Quercus robur and Q. petraea in a mixed oak stand in Denmark. Annals of Forest Science, 66, 706-706. https://doi.org/10.1051/forest/2009058
Kremer A, Hipp AL. (2020). Oaks: an evolutionary success story. New Phytologist, 226(4), 987-1011. https://doi.org/10.1111/nph.16274
Larsen JB ,Jensen JS, Møller IS (1997). EG. Stilkeg og Vintereg – arts og proveniensvariation, forædling og frøkildevalg. Dansk Skovbrugs Tidsskrift, 92-97.
Leroy T, Louvet JM, Lalanne C, Le Provost G, Labadie K, Aury JM, Delzon S, Plomion C, Kremer A. (2020). Adaptive introgression as a driver of local adaptation to climate in European white oaks. New Phytologist, 226(4), 1171-1182. https://doi.org/10.1111/nph.16095
Leroy T, Rougemont Q, Dupouey J-L, Bodénès C, Lalanne C, Belser C, Labadie K, Le Provost G, Aury J-M, Kremer A, et al. (2020). Massive postglacial gene flow between European white oaks uncovered genes underlying species barriers. New Phytologist, 226(4), 1183-1197. https://doi.org/10.1111/nph.16039
Leroy T, Roux C, Villate L, Bodénès C, Romiguier J, Paiva JAP, Dossat C, Aury JM, Plomion C, Kremer A. (2017). Extensive recent secondary contacts between four European white oak species. New Phytologist, 214(2), 865-878. https://doi.org/10.1111/nph.14413
Löf M, Brunet J, Hickler T, Birkedal M, Jensen A. (2012). Restoring Broadleaved Forests in Southern Sweden as Climate Changes. In: Stanturf J, Madsen P, Lamb D. (eds) A Goal-Oriented Approach to Forest Landscape Restoration. World Forests, 16. Springer, Dordrecht. https://doi-org.proxy.lnu.se/10.1007/978-94-007-5338-9_14
Mauri A, Girardello M, Strona G, Beck PSA, Forzieri G, Caudullo G et al. (2022). EU-Trees4F, a dataset on the future distribution of European tree species. Scientific Data 9(37), 1-12. https://doi.org/10.1038/s41597-022-01128-5
Milesi P, Kastally C, Dauphin B, Cervantes S, Bagnoli F, Budde KB, Cavers S, Fady B, Faivre-Rampant P, González-Martínez SC, et al. (2023). Resilience of genetic diversity in forest trees over the Quaternary. bioRxiv:2023.2001.2005.522822.
Olofsson E, & Jakobsson R. (2023). The potential to develop environmental values on privately owned forest land in southern Sweden. Scandinavian Journal of Forest Research, 38(5), 300–315. https://doi.org/10.1080/02827581.2023.2225871
Petit RJ, Bodénès C, Ducousso A, Roussel G, Kremer A. (2004). Hybridization as a mechanism of invasion in oaks. New Phytologist, 161(1), 151-164. https://doi.org/10.1046/j.1469-8137.2003.00944.x
Petit RJ, Brewer S, Bordács S, Burg K, Cheddadi R, Coart E, Cottrell J, Csaikl UM, van Dam B, Deans JD, et al. (2002). Identification of refugia and post-glacial colonisation routes of European white oaks based on chloroplast DNA and fossil pollen evidence. Forest Ecology and Management, 156(1-3), 49-74. https://doi.org/10.1016/S0378-1127(01)00634-X
Petit RJ, Csaikl UM, Bordacs S, Burg K, Coart E, Cottrell J, van Dam B, Deans JD, Dumolin-Lapegue S, Fineschi S, et al. (2002). Chloroplast DNA variation in European white oaks phylogeography and patterns of diversity based on data from over 2600 populations. Forest Ecology and Management, 156, 5-26.
Ruotsalainen S, Himanen K, Viherä-Aarnio A, Aarnio L, Haapanen M, Luoranen J, Matala J, Riikonen J, Uotila K, Ylioja T (2022) Puulajivalikoiman monipuolistaminen metsänviljelyssä: Synteesiraportti. Luonnonvara- ja biotalouden tutkimus; Numero 24/2022; Sivut 135 s.; http://urn.fi/URN:ISBN:978-952-380-394-7
Sáenz-Romero C, Lamy JB, Ducousso A, Musch B, Ehrenmann F, Delzon S, Cavers S, Chalupka W, Dagdas S, Hansen JK, et al. (2017). Adaptive and plastic responses of Quercus petraea populations to climate across Europe. Global Change Biology, 23(7), 2831-2847. https://doi.org/10.1111/gcb.13576
Skrøppa T, Fjellstad KB & Hansen JK. (2017). Genetisk variasjon mellom norske populasjoner av eik. Resultater fra forsøk i Norge og Danmark. (NIBIO Rapport 3(69)). Retrieved from http://hdl.handle.net/11250/2446670
Solvin T, Fløistad IS, Prochowsky GF, Leisgaard T, Ylioja T, Tynkkynen M, Skúlason B, Björgvinsson HS, Stokke E, Myhre MF, Edvardsson E, Uggla C. (2023). Statistics: Forest Seeds and Plants in the Nordic Region 2023. (NordGen Publication Series 2023:02). Retrieved from https://publication.nordgen.org/Forest-Seeds-and-Plants-Statistics-v2023/index.html